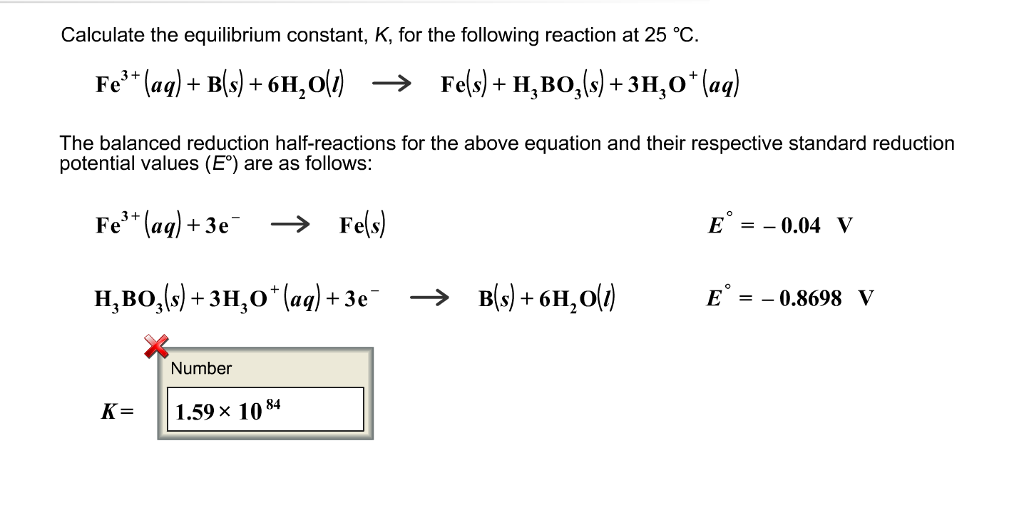
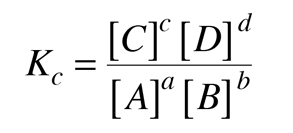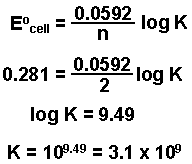SOLVED: Using any data you can find in the ALEKS Data resource, calculate the equilibrium constant K at 25.0 %€ for the following reaction. C(s) 2 Clz(g) CCI(g) Round your answer to
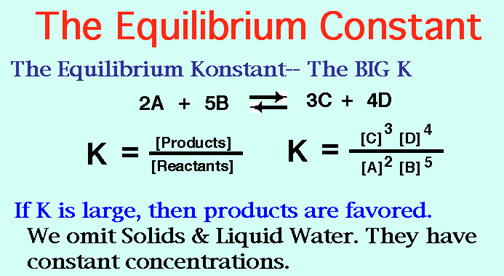
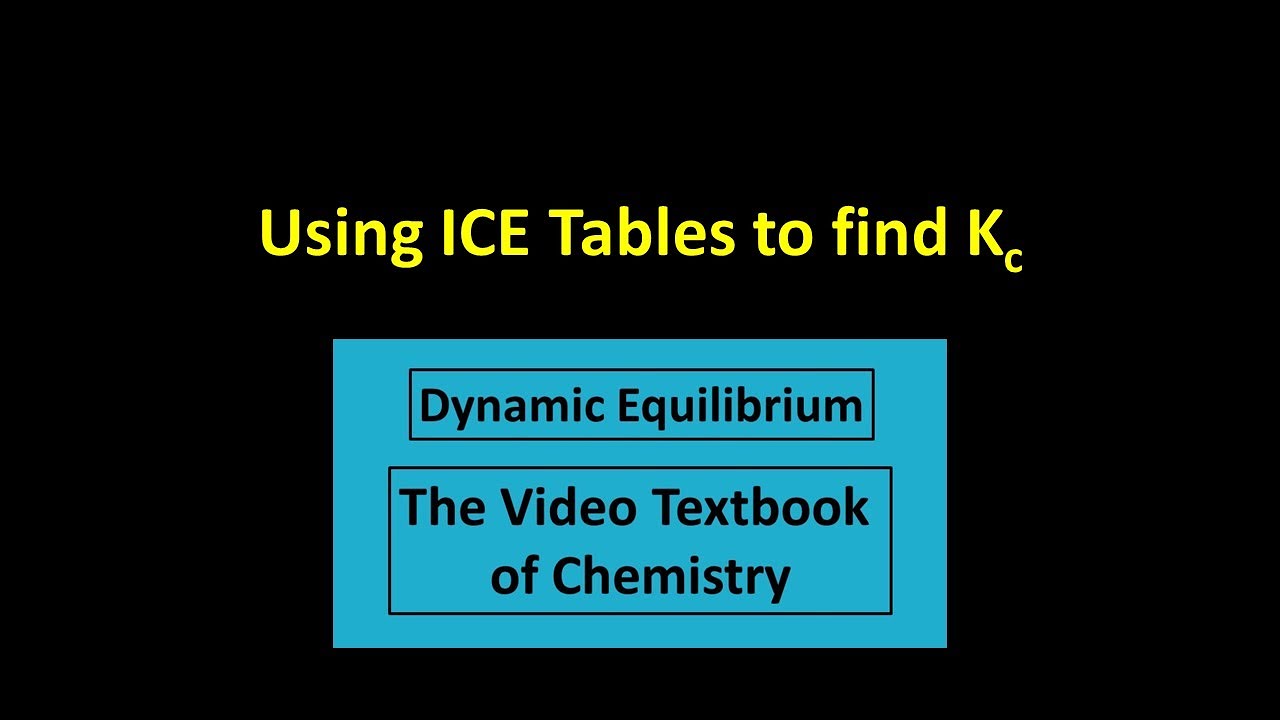
How does the value of an equilibrium constant relate to the relative quantities of reactants and products at equilibrium? | Socratic

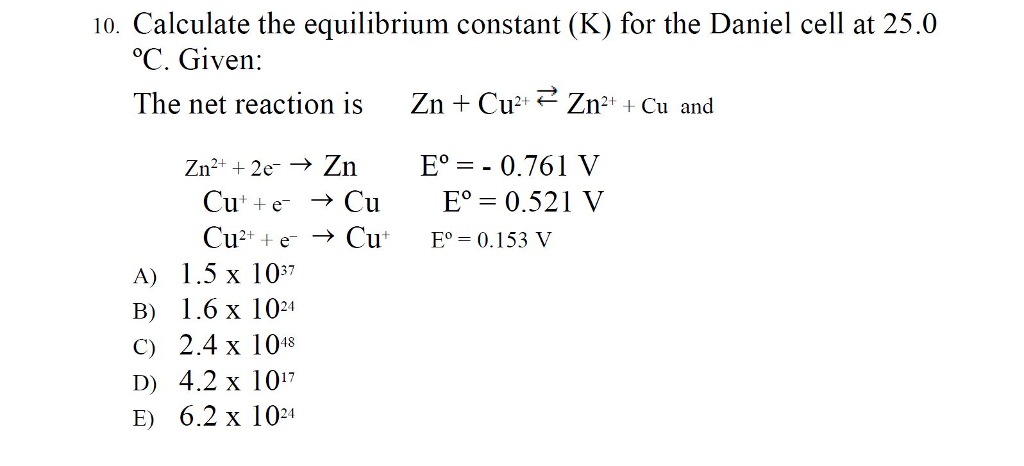
Calculate the equilibrium constant for the reaction, at 25^oC Cu(s) + 2Ag^ + (aq) → Cu^+2 (aq) + 2Ag (s)at 25^oC , E^o cell = 0.47 V, R = 8.134 JK^-1 F = 96500 C is
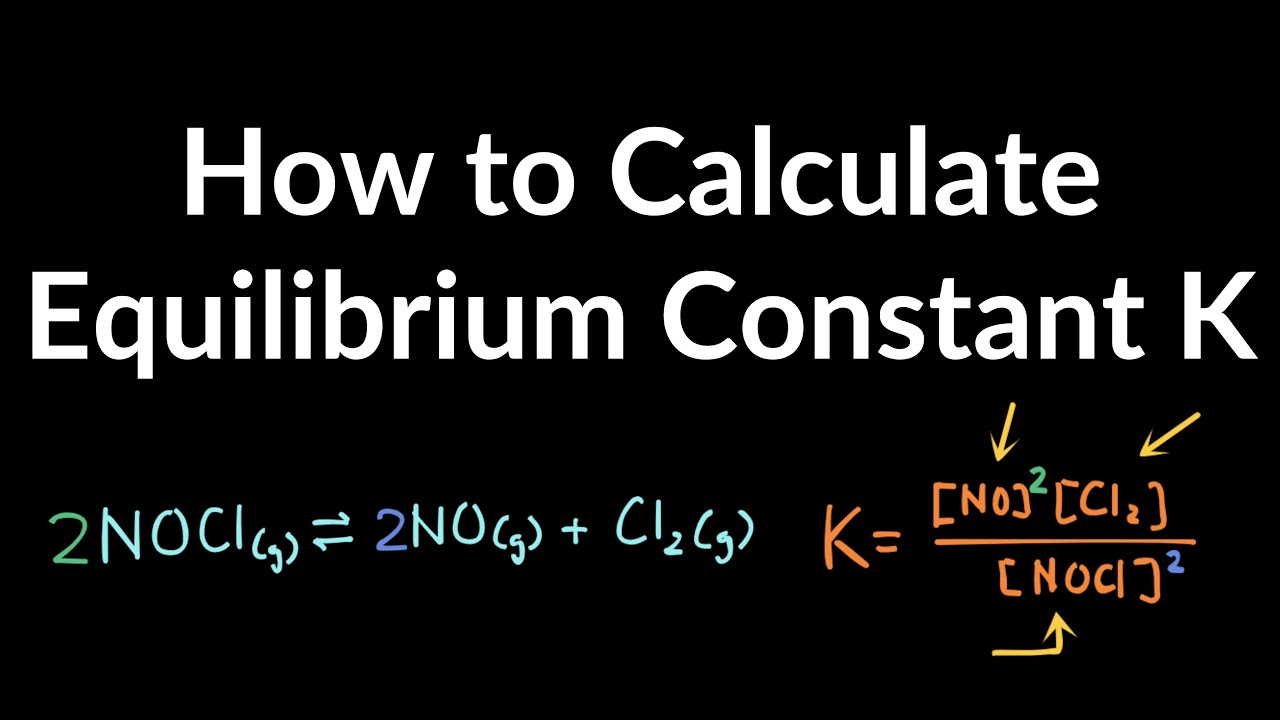
How to Calculate Equilibrium Constant K Value Practice Problems & Exampled Explained Step by Step - YouTube

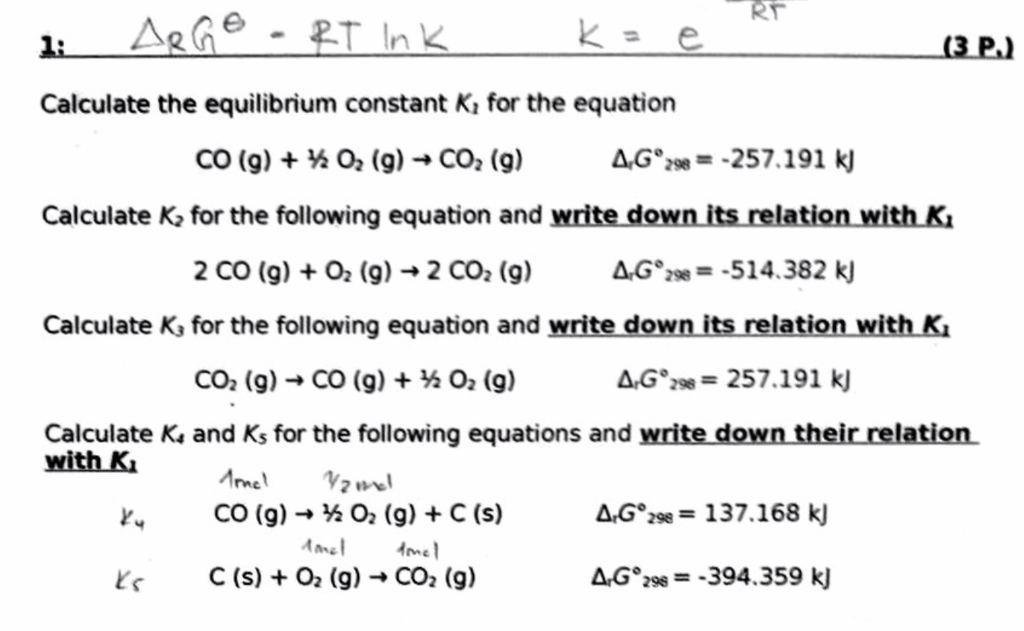
SOLVED: 15.27 The following equilibria were attained at 823 K: Co(s) + H,O(g) K = 67 CoO(s) + Hz(g) Co(s) + COz(g) Kc 490 CoO(s) + CO(g) Based on these equilibria, calculate