Table 5 from An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar | Semantic Scholar

Table 6 from An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar | Semantic Scholar
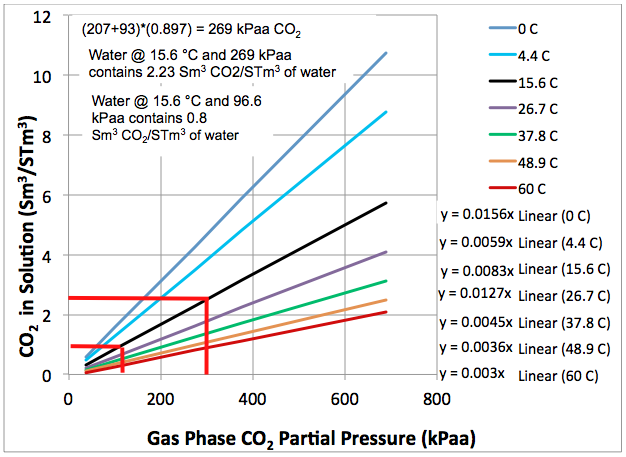
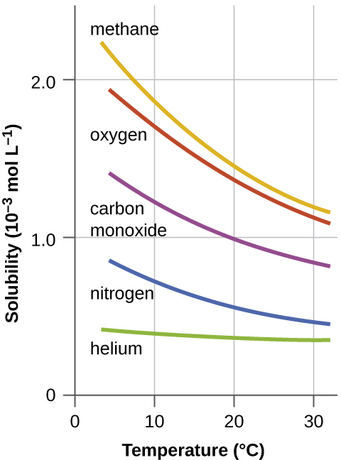
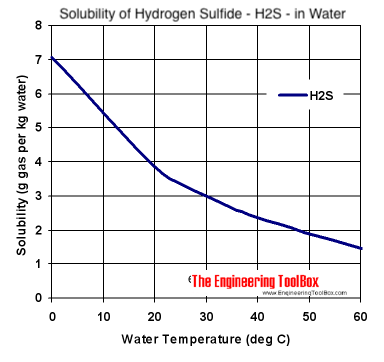
CO2 Flashing from Water is Important for CO2 EOR Flood Separators and Tanks | Campbell Tip of the Month

CO2 solubility measurements in brine under reservoir conditions: A comparison of experimental and geochemical modeling methods - Steel - 2016 - Greenhouse Gases: Science and Technology - Wiley Online Library
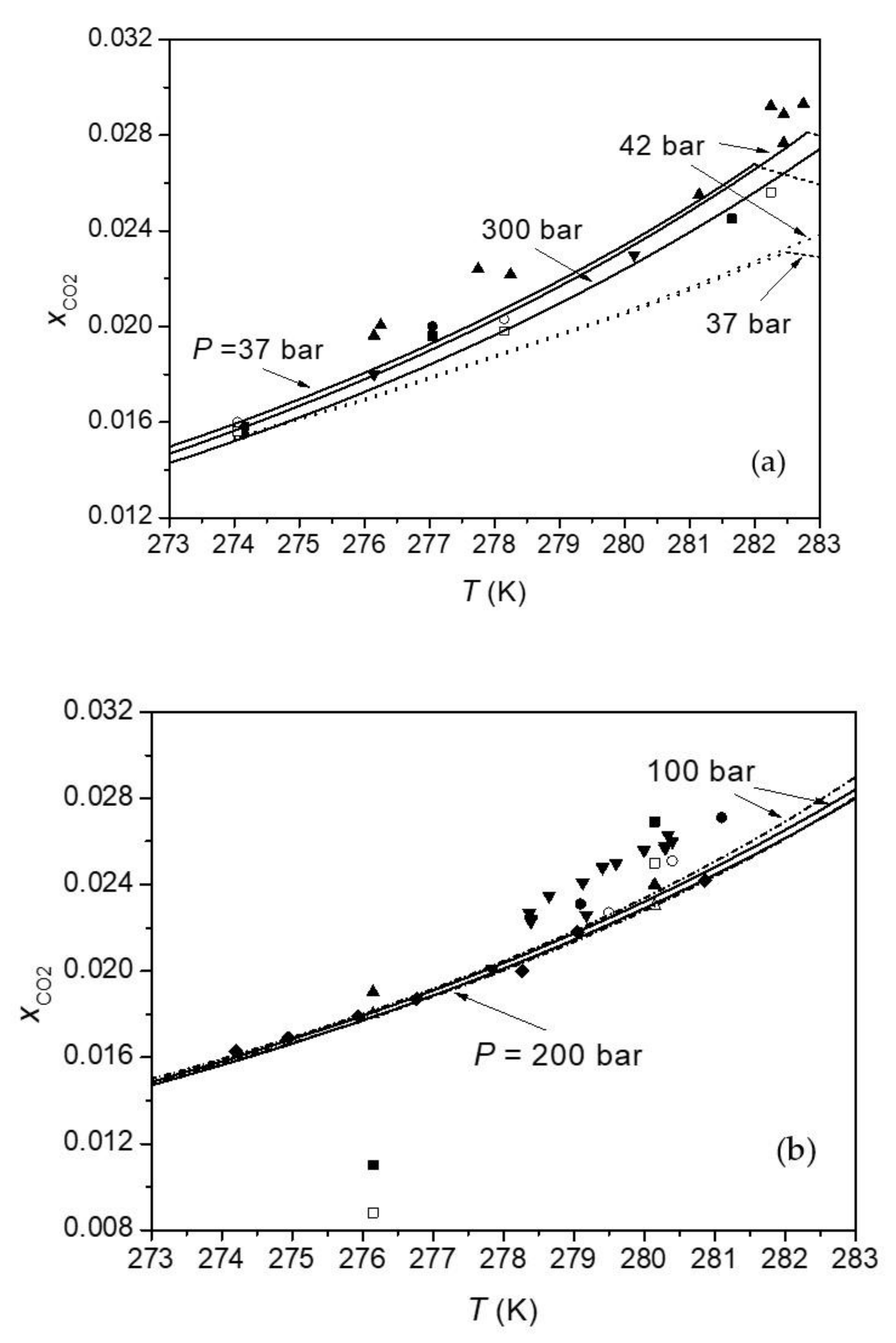
Minerals | Free Full-Text | An Accurate Model to Calculate CO2 Solubility in Pure Water and in Seawater at Hydrate–Liquid Water Two-Phase Equilibrium
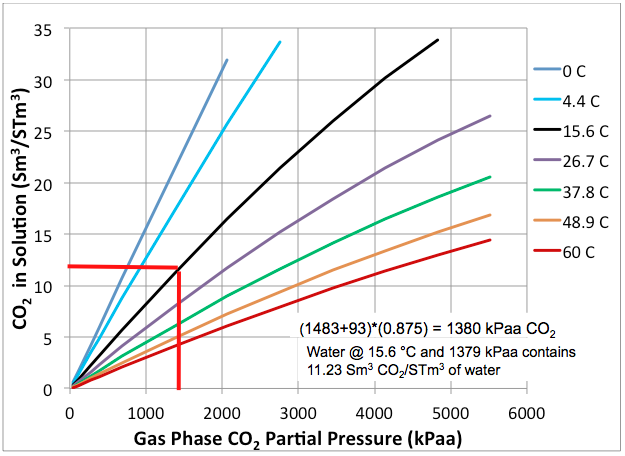
CO2 Flashing from Water is Important for CO2 EOR Flood Separators and Tanks | Campbell Tip of the Month

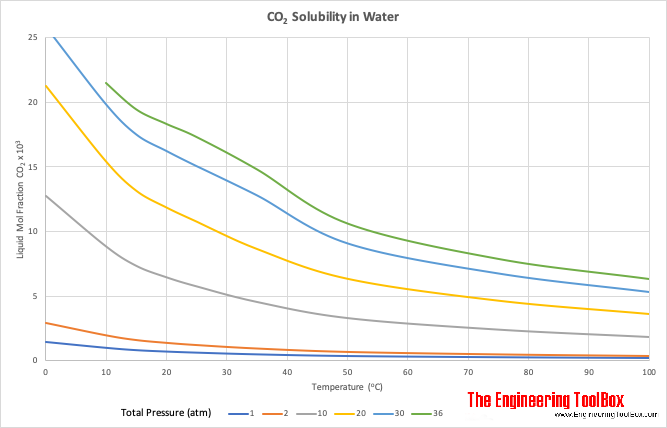
Solubility of CO2 in water from −1.5 to 100 °C and from 0.1 to 100 MPa: evaluation of literature data and thermodynamic modelling - ScienceDirect

An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar - ScienceDirect

Table 3 from An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar | Semantic Scholar
![State Henry's law. Calculate the solubility of CO2 in water at 298 K under 760 mm Hg. [K(H) for CO2 in water at 298K is 1.25 xx 10^(6) mm Hg] State Henry's law. Calculate the solubility of CO2 in water at 298 K under 760 mm Hg. [K(H) for CO2 in water at 298K is 1.25 xx 10^(6) mm Hg]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/571227035_web.png)